In a small lab in Luxembourg, German neuroscientist Jens Schwamborn is reprogramming human cells to create miniature 3D brains. His goal? To crack Parkinson’s disease and deliver personalised treatments that the world has been waiting for, all without relying on traditional animal models.
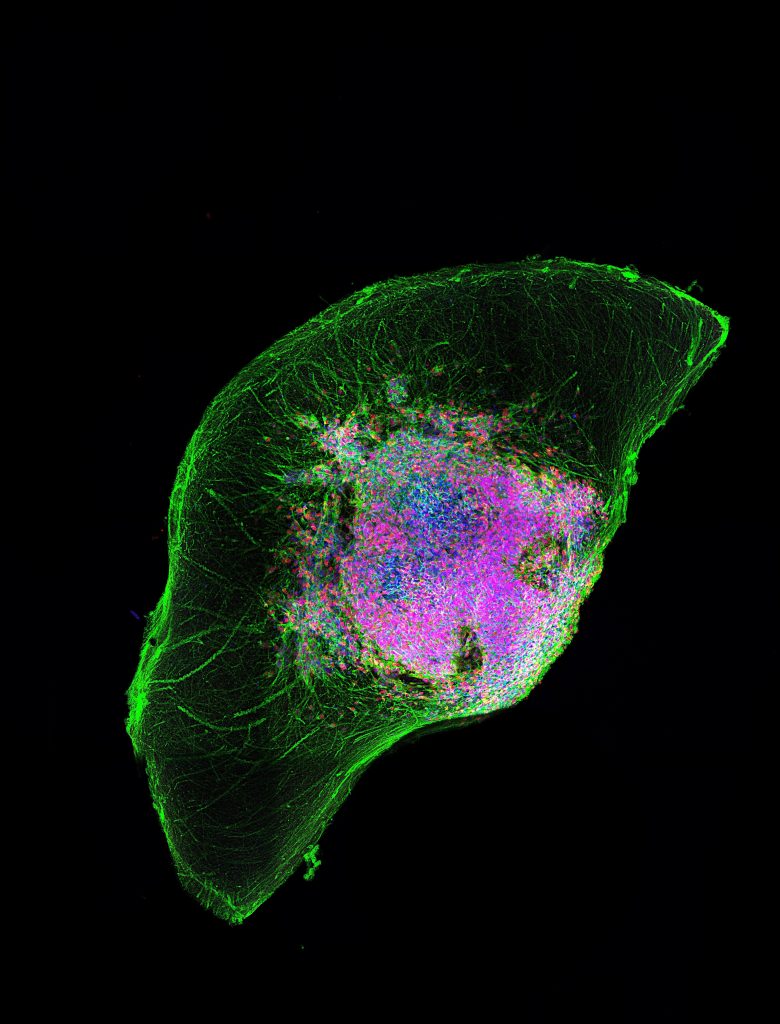
By reprogramming human cells, OrganoTherapeutics recreates genuine 3D models of the human brain in the laboratory. These models make it possible to study the neurodegeneration associated with Parkinson’s disease and to accelerate the discovery of targeted therapies.
It all began in 2013, when Jens Schwamborn, a professor of cell biology and future CEO of OrganoTherapeutics, joined the University of Luxembourg. Together with two members of his team, he built upon the work of 2012 Nobel Prize winner Shinya Yamanaka, who discovered how to reprogramme adult cells into induced pluripotent stem cells (iPS cells), undifferentiated cells which are capable of becoming almost any type of cell.

By using a patient’s skin or blood cells, it becomes possible to preserve their genetic heritage and recreate the cells affected by the disease, such as those impacted by Parkinson’s, in order to study its progression and test personalised treatments. The shift to a 3D model also enables a far more accurate reproduction of the organisation and interactions of human tissues than traditional 2D cell cultures. And that is precisely what OrganoTherapeutics offers.
“We have developed a method to guide these stem cells to differentiate into a specific region of the brain, in this case, the midbrain, which is affected by Parkinson’s disease,” explains Prof Schwamborn. “By creating these brain organoids from a patient’s cells, we can observe characteristics identical to those of the actual disease. And if we can do that, we can also develop new therapies. That’s why we started.”
Currently available treatments do not yet halt or even slow the progression of Parkinson’s disease, and research based on standard cell cultures or animal models has, so far, proved ineffective.
Losing pitches but gaining visibility
Seeing commercial potential in their technology, the researchers filed a patent and founded OrganoTherapeutics in 2019. They devoted the first year to pitching to potential investors. “That completely failed, it was considered too risky, too early, the usual reasons,” recalls Prof Schwamborn.
However, the exercise was not in vain. It gave OrganoTherapeutics greater visibility. To the point that a pharmaceutical company reached out, not to invest directly, but to test its own compounds using the start-up’s technology.
“That went well, they were satisfied and have now been a regular client for four or five years,” says Prof Schwamborn. “We realised that this business model could work, so we approached other companies, and more firms started contacting us.”
“We realised that this business model could work, so we approached other companies, and more firms started contacting us.”
Today, OrganoTherapeutics has a portfolio of clients, some regular, others for one-off projects. Among them are pharmaceutical and biotechnology companies of all sizes, from small local players to multinationals, from around the world, notably Japan, Europe, and the United States.
Client-based and public funding model
Having reached profitability, OrganoTherapeutics now funds itself through its clients, along with some research grants from the UK and the US, as well as financing from the European Union and the Luxembourg government. The startup was also recently accepted into a programme from the Michael J. Fox Foundation, one of the leading organisations supporting Parkinson’s research.
Demand for innovative solutions is strong: by 2050, an estimated 25.2 million people worldwide will be living with Parkinson’s disease, an increase of 112% compared with 2021, mainly due to population ageing.
Last November, the US Food and Drug Administration (FDA) also announced its intention to reduce animal testing in drug development. “There is therefore an enormous need for alternative models, and our technology meets that demand perfectly,” emphasises Prof Schwamborn.
Expanding to other neurogenerative disorders
With its team of seven employees, OrganoTherapeutics is now focusing on two main goals: improving the quality, complexity, and predictive capability of its 3D models by building a vast database of phenotypes and leveraging it through deep learning. At the same time, the startup aims to extend its model, initially dedicated to Parkinson’s disease, to other neurodegenerative disorders such as schizophrenia, Huntington’s disease, and Alzheimer’s.
“We are becoming a precision medicine service provider,” summarises Prof Schwamborn, who envisions a scenario for the coming years: “The idea is that when a patient is newly diagnosed with Parkinson’s disease or another condition, we can create a personalised brain organoid and test all available treatments to indicate to the doctor which medication is most suitable.”
And perhaps then, truly effective treatments against Parkinson’s disease will finally exist.

This article was published in the 8th edition of Forbes Luxembourg.
Read more articles:
UNICITY: Redefining Urban Value In Luxembourg’s Gare District

